Turning cutting edge bioelectronic concepts into clinical reality
When considering a new bioelectronic technology, there are many important questions that innovators need to answer. What problem are you trying to solve? Is it safe? Is it effective? Can it be used clinically? Who is paying?
With patient safety at its core, the MedTech sector is complex, multi-disciplinary and highly regulated. For investors, it is high-risk and does not present a ‘get-rich-quick' opportunity.
In this webinar, Ludovic Labat (CEO, Neo-Bionica), Lusia Guthrie (Chair, Neo-Bionica & Founder, LBT Innovations) and Owen Burns (Head of R&D, Neo-Bionica) will share their expert insights into what is required to commercialise MedTech research, taking an academic concept through to product launch. Using real-world technology case studies examples, they will highlight how researchers can capitalise on market opportunities within a limited window of time. Learn about how Neo-Bionica are working to reduce the risk involved in the path to commercialisation and saving costs through partnership with world-class entrepreneurs, scientists, engineers and clinicians.
About Neo-Bionica:
Neo-Bionica was established in 2021 and is a joint venture between Bionics Institute and the University of Melbourne. The venture is backed by 35 years of innovation in medical devices, including the Cochlear implant, the Minder® device and the Vagal Nerve Stimulation device. Neo-Bionica own and operate a world-class cleanroom facility located within the St Vincent’s Hospital campus.
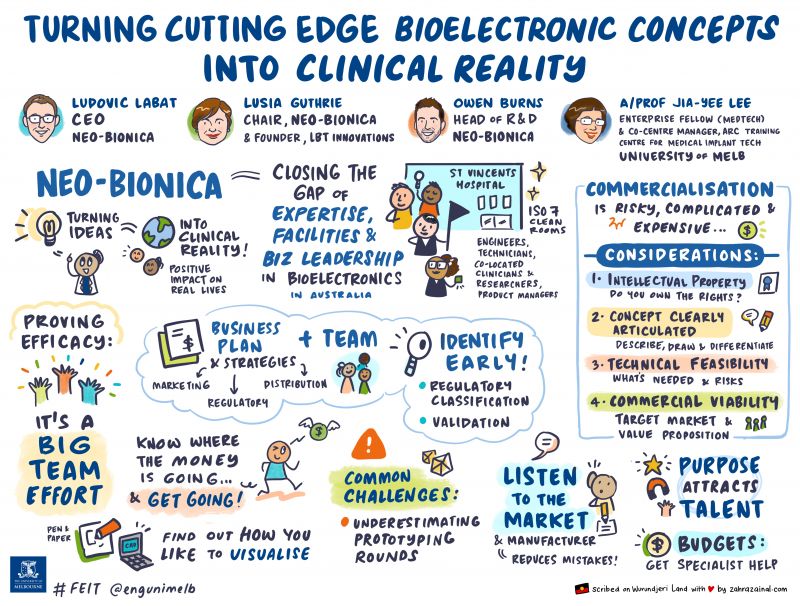
Introducing our speakers and chair:
Ludovic Labat (Keynote speaker)
CEO, Neo-Bionica
As a business leader, Ludovic has over 20 years’ experience in the medical device and technology industry in Europe, the USA and Asia Pacific with a range of global companies including Fortive, Danaher, Siemens, Tektronix and Invetech. He has a record of proven success in bringing innovations to market to generate profitable growth in global hi-tech and medical devices markets – in roles ranging from product ideation to go-to-market strategy.
Lusia Guthrie
Chair, Neo-Bionica & Founder, LBT Innovations
Lusia is a medtech entrepreneur with over 35 years in the pharmaceutical and bioscience industries. She has extensive experience in forming companies, raising capital, developing products and bringing them to market. Lusia founded LBT Innovations to bring advanced technologies to the medical industry – and listed the company on the Australian stock exchange.
She also led the company’s development of an automated plate assessment system for microbiologists, which was the first artificial intelligence diagnostic medical device to achieve US FDA class II approval.
Owen Burns
Head of R&D, Neo-Bionica
An expert systems engineer, Owen is a Head of R&D at Neo-Bionica and Co-Head of the Bionics Institute’s Engineering capability.
His work has focused on mechanical and systems engineering focusing on implantable neuro-modulation devices, with expertise in in bio-safe materials and bio-mechanical engineering.
Owen was the lead design engineer for the MinderTM electrode, currently in first-inhuman trials, and the vagus nerve stimulation device developed to treat Crohn’s disease, which is due to start first-in-human trials soon.
Associate Professor Jia-Yee Lee (Chair)
Enterprise Fellow (Medtech) and Co-Centre Manager, ARC Training Centre for Medical Implant Technologies, Faculty of Engineering and Information Technologies, University of Melbourne .
Associate Professor Jia-Yee Lee is the Enterprise Fellow for Medtech and Co-Centre Manager at the ARC Training Centre for Medical Implant Technologies (ARC CMIT), Faculty of Engineering and Information Technologies, University of Melbourne. Jia-Yee is recognised for her expertise in medical research, industry engagement, technology start-up, commercialisation and policy development. Jia-Yee is a passionate advocate for venture creation and brings hands on experience in working with clinicians, engineers, entrepreneurs and industry to commercialise research. She is a Member of the Steering Committee, BridgeTech Program, that is hosted by Queensland University of Technology. Her role at the Victorian Department of Health has resulted in the development of the Government’s Health and Medical Research Strategy 2016-2020. As an experienced technologist she has delivered large scale programs such as the Victorian electronic Clinical Systems that is now implemented in several public hospitals. Her contributions to research include leading engineers, computer scientists and mathematicians to drive innovation in Diagnostic Genomics, Assistive Technologies and Biomedical Informatics at National ICT Australia, now part of CSIRO Data61. Jia-Yee has spent close to 20 years in medical research having led NHMRC funded projects at the Victorian Infectious Diseases Reference Laboratory and Burnet Institute. She has an MBA from the Melbourne Business School and a PhD in virology from the University of Melbourne.